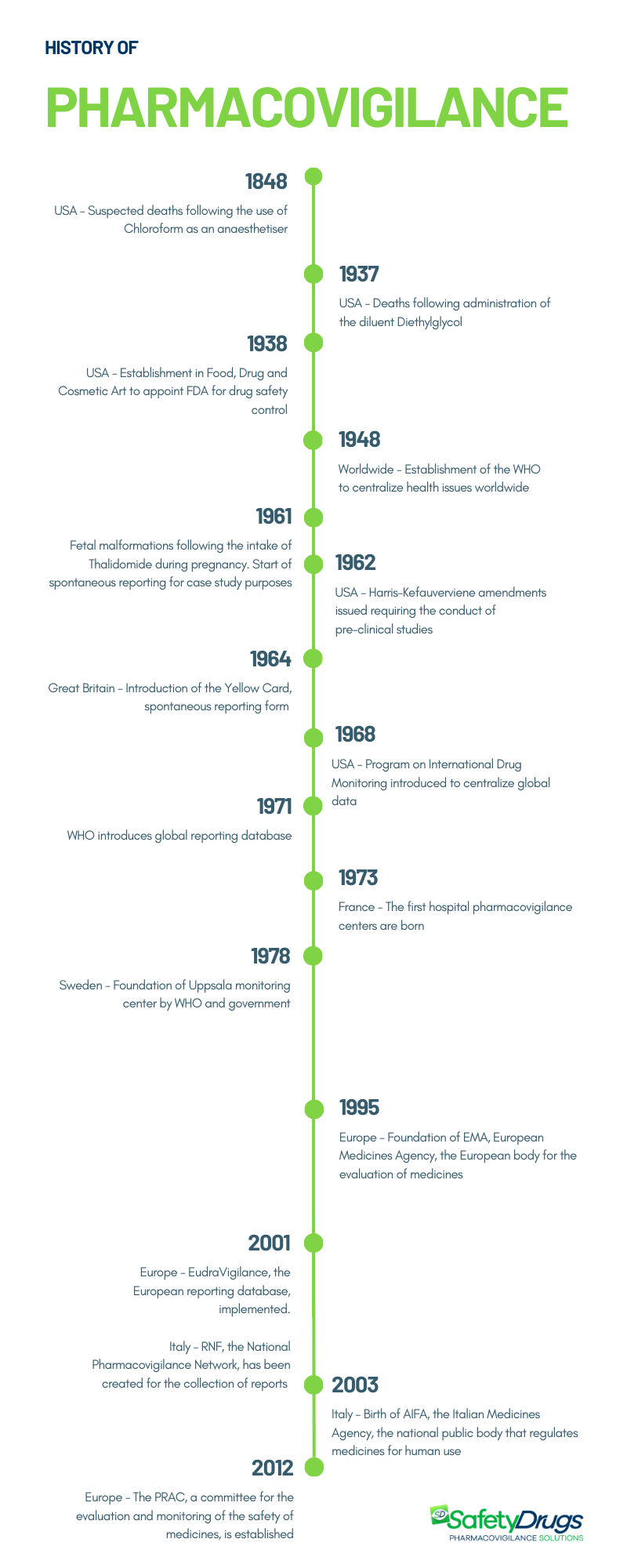
Pharmacovigilance was officially born in 1961 in Great Britain following the serious adverse events caused by the administration of Thalidomide to pregnant women. However, numerous events have marked the history of pharmacovigilance. Here is its evolution, from its origin in 1848 with the use of Chloroform, to its current form.
What is Pharmacovigilance?
The term pharmacovigilance derives from the Greek and Latin words pharmakon, meaning medicinal substance, and vigila, meaning to watch. Pharmacovigilance, according to the World Health Organization (WHO), is “the discipline and set of activities aimed at the identification, evaluation and prevention of adverse effects or other problems related to the use of medicines”. It is therefore a fundamental activity to guarantee the safety profile of a drug.
You can learn more by reading: Pharmacovigilance: how it works
When was pharmacovigilance born?
Pharmacovigilance was born in 1961 in Great Britain, when, following the administration of Thalidomide to pregnant women, numerous episodes of fetal malformations occurred. This crucial event marked the start of drug surveillance activity, but there were numerous cases of previous suspected reactions that led to this activity as we know it today. The first occurred in 1848.
History of pharmacovigilance: key events
As previously mentioned, over the years there have been various events that have led to the establishment of modern pharmacovigilance. Here they are below.
1848 – Chloroform
The history of pharmacovigilance dates back to 1848, when a series of suspicious deaths occurred in Great Britain during operations in which chloroform was administered to patients as an anesthetic.
Chloroform was used as an anesthetist starting in 1847. The following year a 15-year-old girl died following its use. This tragic event sparked concern, prompting the Lancet Journal to set up a commission and urge British doctors to report similar cases. A debate therefore opened on the safety of anesthetic procedures and following various reports, the drug ceased to be used as an anesthetic in 1976. This episode represented the first step towards the establishment of pharmacological safety procedures.
1937 – Sulfanilamide
Another turning point occurred in the United States in 1937, when 107 people, including 76 newborns, lost their lives due to a new liquid formulation of Sulfanilamide containing the solvent Diethylglycol as a diluent, which caused fatal reactions. Currently, this component is known for its high toxicity and is used as an antifreeze liquid in car engines. This tragedy highlighted the importance of ensuring the safety not only of the active ingredient, but also of the excipients that make up the drug.
1961 – Talidomide
The decisive event for the birth of pharmacovigilance occurred in 1961 when the use of Thalidomide during pregnancy caused a 20% increase in congenital malformations in newborns. The drug was tested for two years on 300 patients, without detecting any particular side effects. Therefore, considered safe, it was marketed in over 50 countries starting from 1957. Thalidomide was used primarily as a sedative, antiemetic and hypnotic in pregnant women. Its administration caused a serious anomaly in the development of the fetus: the newborns had serious deformities of the limbs, especially the upper ones, such as the absence (amelia) or reduction of the bones (phocomelia). Approximately 10,000 to 20,0000 children suffered incomplete development.
In 1961, reports on possible correlations between Thalidomide and congenital malformations were published in the scientific journal Lancet. The turning point came with the letter from Doctor William Griffith McBride in December of the same year, in which he suggested a connection between congenital malformations and the intake of the drug and making public the first cases of fetal abnormalities linked to Thalidomide. This can be considered as the beginning of spontaneous reports. The hypothesis of the correlation between the malformations and the intake of the drug was therefore consolidated, and it was withdrawn from the market. This event in the history of pharmacovigilance marked the transition from an occasional activity to a systematic, organized and regulated process.
You can discover more reading Thalidomide: the pharmaceutical scandal that changed pharmacovigilance forever
History of pharmacovigilance: birth of the bodies
Following these events, the first bodies and procedures emerged aimed at monitoring the safety of the drug.
In 1938 the Food, Drug and Cosmetic Act (FFDCA, FDCA, or FD&C) was established in the United States, a set of laws to charge the Food and Drug Administration (FDA) (established in 1906) with supervising the safety of foods, drugs, medical devices and cosmetics, laying the foundations of pharmaceutical legislation.
In 1948, the World Health Organization (WHO) was founded in Geneva to centralize health issues worldwide.
In 1962, following the Thalidomide accident, the Harris-Kefauver amendments were introduced in the United States, which mandated the need to conduct mandatory preclinical studies. Only after the evaluation of the results of these studies was it possible to start the clinical trial phase on humans. Furthermore, the marketing authorization depended on the data obtained in the three phases of the clinical trial. After marketing, the drugs were subjected to post-marketing surveillance.
In the United Kingdom, the Yellow Card, the first form for reporting adverse reactions by doctors, was introduced in 1964.
The WHO, in 1968, promoted the Program on International Drug Monitoring (PIDM), an international drug monitoring program aimed at centralizing global data on adverse reactions. 10 states were initially involved: Australia, Canada, Czechoslovakia, Ireland, the Netherlands, Germany, New Zealand, Sweden, the United Kingdom, the USA (Italy joined in 1975). The program was immediately effective: the following year it emerged that Clioquinol, an anitimycotic drug, caused retrobulbar optic neuritis in Asian countries, revealing an ethnic susceptibility to the drugs and their adverse effects. Furthermore, in 1971 it emerged that diethylstilbestrol, an estrogen of synthetic origin, which had been administered since the 1940s to pregnant women to prevent spontaneous abortions and to relieve nausea, caused genital tumors in the daughters of women exposed to this substance during pregnancy. pregnancy. For pharmacovigilance it meant learning that adverse drug effects can occur years later or even in the next generation. In that same year, WHO created the global reporting database located in Uppsala, Sweden.
In 1973, France inaugurated the first six hospital surveillance centers, where the term pharmacovigilance was formally adopted.
The Swedish government and the WHO founded the Uppsala monitoring center in 1978.
In 1995, the European Medicines Agency (EMA) was founded. In 2001, Eudravigilance, the European database for the management of reports, was implemented.
In Italy, the National Pharmacovigilance Network (RNF) was created in 2001 to collect reports of adverse reactions at a national level and in 2003 the Italian Medicines Agency (AIFA) was created. As proof of the growing awareness at a national level, some collaborative groups were established such as the Interregional Pharmacovigilance Group (GIF) and the Italian Group for Epidemiological Studies in Dermatology (GISED). Furthermore, since 2006, Italian healthcare companies have been obliged to designate a qualified person as responsible for pharmacovigilance activities (QPPV).
With the establishment of the Pharmacovigilance Risk Assessment Committee (PRAC) in 2012, the European pharmacovigilance system was further strengthened, clearly defining roles and responsibilities. The PRAC is responsible for evaluating and monitoring the safety of medicines for human use, providing recommendations to the relevant committees.
History of pharmacovigilance: timeline
History of pharmacovigilance: the current scenario
Pharmacovigilance has made significant progress, moving from a passive monitoring system that relied exclusively on spontaneous reporting of suspected adverse reactions to a more proactive approach. The latter makes use of targeted tools and procedures which include detailed risk monitoring planning (Risk Management Plan). Pharmacovigilance regulations have played a key role in regulating the activities of the various figures involved and recent legislative updates have broadened the definition of adverse reactions to include medication errors, abuses and overdoses, thus leading to an increase in reporting. European regulations now also require greater timeliness in reporting.
The evolution of pharmacovigilance has been made possible above all thanks to the improvement of both the quantity and quality of spontaneous reports, actively involving professionals and patients. Furthermore, activities aimed at refining signal search methods and improving communication with healthcare professionals were conducted. Pharmacovigilance systems around the world are undergoing significant changes in line with technological progress, such as the transition to ICH E2B (R3), the recent electronic reporting format – Individual Case Safety Report (ICSR). An essential help for all operators involved in pharmacovigilance processes is to equip themselves with a safety database developed in compliance with the most recent regulatory requirements.







