The coding of adverse events is a crucial step in pharmacovigilance: selecting the correct term ensures that data are clear, comparable, and useful for identifying safety signals. MedDRA is the international standard that enables this process. In this article, we explore its structure, hierarchical logic, and operational importance for professionals in the field.
What is MedDRA?
MedDRA (Medical Dictionary for Regulatory Activities) is the standardized medical-regulatory terminology used internationally to code, classify, and analyze information related to adverse events, adverse reactions, medical conditions, clinical test results, and device malfunctions.
MedDRA terminology was introduced in the 1990s by the ICH (International Council for Harmonisation) to harmonize international communication in the medical regulatory field. Before that, the sector relied on multiple terminological systems such as WHO-ART© (World Health Organization’s Adverse Reaction Terminology), ICD-9 (International Classification of Diseases Ninth Revision), and COSTART© (FDA’s Coding Symbols for a Thesaurus of Adverse Reaction Terms). This fragmentation made data retrieval, cross-referencing, and analysis difficult.
The adoption of MedDRA overcame these limitations by offering a common, precise, and constantly updated language. The first version, MedDRA Version 1.0, was developed using the terminology of the British MCA authority (now MHRA – Medicines and Healthcare products Regulatory Agency). The second version, MedDRA Version 2.0, was the first officially adopted and implemented version.
Where is MedDRA applied?
MedDRA terminology is used throughout the entire lifecycle of medicinal products for human use. In clinical settings, it is applied for coding adverse events and clinical test results in phase I, II, III, and IV studies, ensuring consistency and accuracy in data collection.
In post-marketing pharmacovigilance, MedDRA is the standard for coding spontaneously reported adverse reactions, managing Individual Case Safety Reports (ICSRs), and compiling periodic safety update reports (PSURs/PBRERs). The terminology is also mandatory in regulatory documentation submitted to health authorities (EMA, FDA, PMDA, and others) for marketing authorization applications, variations, and renewals.
MedDRA is used for monitoring and managing adverse events related to medical devices, codifying both malfunctions and their clinical consequences for patients. Additionally, in certain regulatory contexts — such as the monitoring of cosmetics or food supplements subject to surveillance — MedDRA can be employed as a common language for standardizing the description of relevant clinical events and conditions.
MedDRA covers a broad range of medical and regulatory concepts, including symptoms, diseases, diagnoses, therapeutic indications, device malfunctions (e.g., PT Device-related infection, PT Device failure), and even social circumstances relevant to regulatory assessments (e.g., PT Travel abroad or HLT Tobacco use). This breadth makes MedDRA an essential tool for complete and structured data coding.
MedDRA hierarchy structure
One of MedDRA’s strengths is its hierarchical structure, designed to ensure that safety reports and analyses are consistent and easy to navigate. Each term can be explored across different levels of specificity, from broad categories to more detailed terms. The hierarchy consists of five levels: SOC (System Organ Class), HLGT (High-Level Group Term), HLT (High-Level Term), PT (Preferred Term), and LLT (Lowest Level Term).
SOC (System Organ Class) and SOC Groups
SOC represents the highest level of the MedDRA hierarchy and groups terms based on etiology, purpose, and site of manifestation. An example is the SOC “Nervous system disorders,” which includes all terms related to neurological conditions.
Related SOCs are further grouped into thematic categories to enable broader and more complete analyses across related functional areas. In total, there are 27 SOCs, each with a specific role in the classification and analysis of safety data. For example, the SOCs “Renal and urinary disorders” and “Metabolism and nutrition disorders” belong to the same functional group related to metabolic and renal functions.
It is important to note that a single SOC group can be linked to an unlimited number of HLGTs, allowing for a multi-axial view of adverse events.
HLGT (High-Level Group Term)
HLGTs represent the second hierarchical level of MedDRA and organize groups of clinically related events within each SOC. These groupings facilitate data retrieval and enable targeted analysis by therapeutic area or biological function. For example, within the SOC “Nervous system disorders,” the HLGT “Neurological disorders NEC” (not elsewhere classified) groups neurological conditions that are not included in other specific categories. HLGTs play a key role in supporting data presentation and extraction.
Each HLGT must be linked to at least one SOC and one HLT. There are no limits to the number of SOCs an HLGT can be associated with, providing flexibility that reflects the complexity of clinical manifestations.
HLT (High-Level Term)
HLTs constitute the third level of the MedDRA hierarchy and represent specific subgroups within each HLGT. Each HLT serves as an inclusive category that gathers PTs (Preferred Terms) connected by clinical, anatomical, physiological, etiological, or functional affinities.
For example, within the HLGT “Neurological disorders NEC,” we find the HLT “Paresthesias and dysesthesias,” which groups terms related to sensory disturbances such as tingling, altered sensations, and abnormal feelings. This level of detail allows for more precise analysis and structured data organization.
Each HLT must be linked to at least one SOC group through an HLGT. Furthermore, an HLT can only be associated with one HLGT per SOC, ensuring a clear and linear hierarchical path. All HLTs connected to a given HLGT will automatically appear in each SOC to which that HLGT is linked, allowing for a comprehensive view of data.
PT (Preferred Term)
PTs represent the fundamental level for coding adverse events within MedDRA and are the primary coding terms used in pharmacovigilance. They distinctly describe a specific event, such as a symptom, clinical sign, disease, diagnosis, therapeutic indication, investigation, medical or surgical procedure, or relevant family, social, or medical history characteristic.
The PT follows the principle of equivalence, grouping synonyms or closely related terms under a single label, ensuring consistency and clarity in coding and interpretation. An example of a PT is “Burning sensation.”
Each PT must be linked to at least one LLT (Lowest Level Term) and can have an unlimited number of LLTs associated with it, representing terminological variants, synonyms, or abbreviations.
To avoid duplication during data retrieval, each PT is assigned to a primary SOC, although it may appear in multiple SOCs thanks to multi-axiality. This system allows for both coherence and flexibility, accommodating different clinical and regulatory perspectives.
LLT (Lowest Level Term)
LLTs are the most granular and detailed level of the MedDRA hierarchy. They include synonyms, near-synonyms, lexical variants, colloquial or more descriptive forms of the associated PT, and LLTs identical to the PT itself. An example of an LLT is “Burning sensation in limbs,” which allows for the coding of a more specific clinical description reported in a safety case.
This granularity allows reports to be coded exactly as they are described in clinical practice while maintaining consistency and standardization. Each LLT is uniquely linked to a single PT and cannot be associated with multiple terms, ensuring precision and clarity in the coding process.
Below we show an example of a MedDRA hierarchy tree to illustrate the levels. This is a simplified and non-exhaustive example of the MedDRA structure, as each level (SOC, HLGT, HLT, PT, LLT) actually includes hundreds or thousands of terms connected through clinical, anatomical, physiological, and etiological criteria. Furthermore, thanks to multi-axiality, each term can be associated with multiple SOCs, reflecting the complexity of clinical manifestations and the need for cross-sectional and in-depth analyses of pharmacovigilance data.
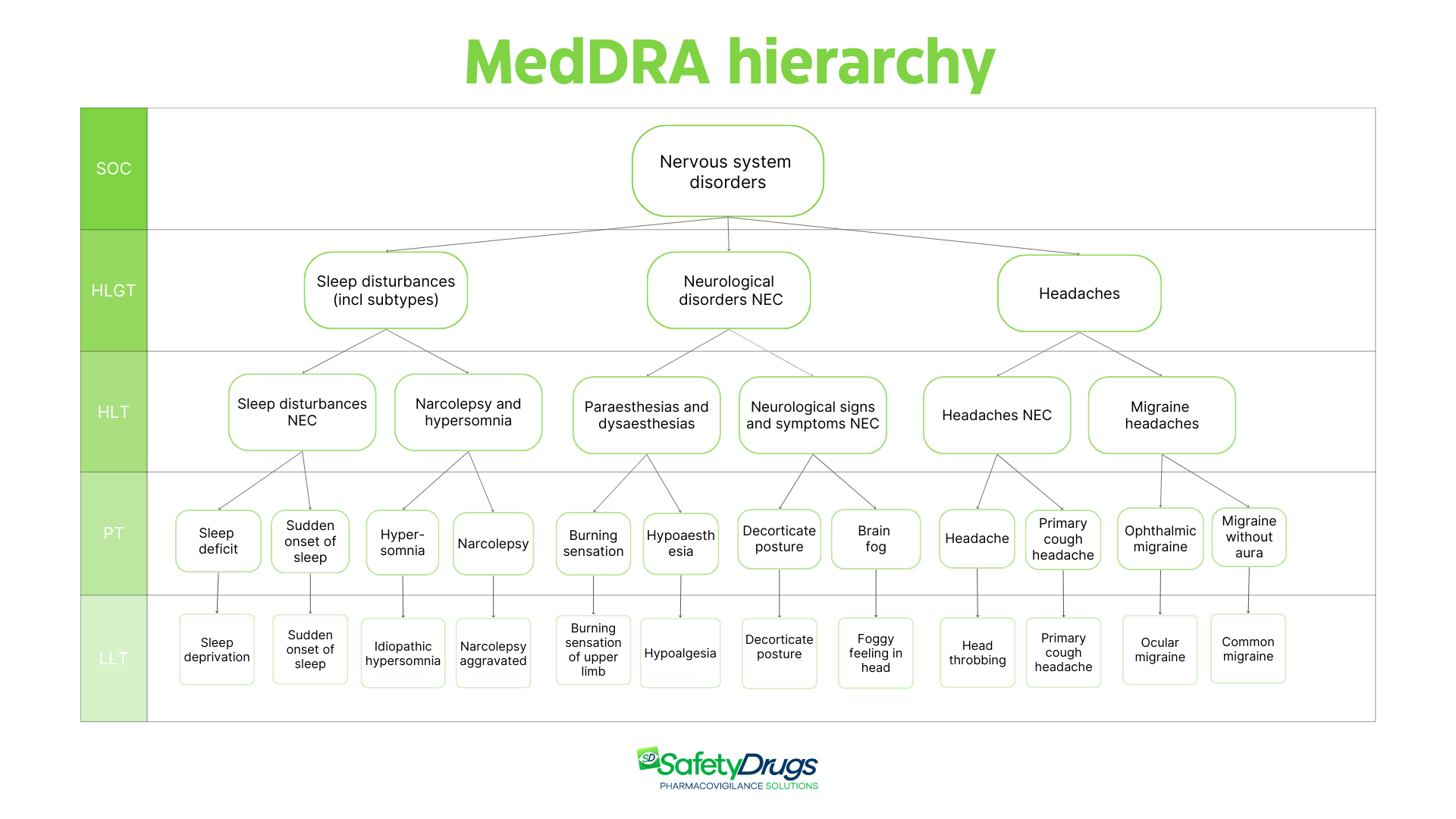
The MedDRA hierarchy represented is a simplified example: each level contains many more terms and, thanks to multi-axiality, a term can be linked to multiple SOCs to reflect the clinical complexity of events. Terms shown are based on MedDRA version 27.1.
What is MedDRA multi-axiality, and why is it important?
As mentioned above, the MedDRA dictionary is characterized by multi-axiality. This feature refers to the possibility that a single term belongs to multiple SOCs, reflecting clinical complexity. Many events cannot be confined to a single SOC, because their clinical manifestations may involve multiple organ systems.
To illustrate the concept of multi-axiality, here is a practical example: the term “Neonatal pulmonary edema” belongs to multiple SOCs: “Respiratory, thoracic and mediastinal disorders,” “Pregnancy, puerperium and perinatal conditions,” and “Cardiac disorders.” This characteristic allows the same event to be analyzed from different perspectives, improving the ability to detect and interpret safety signals.
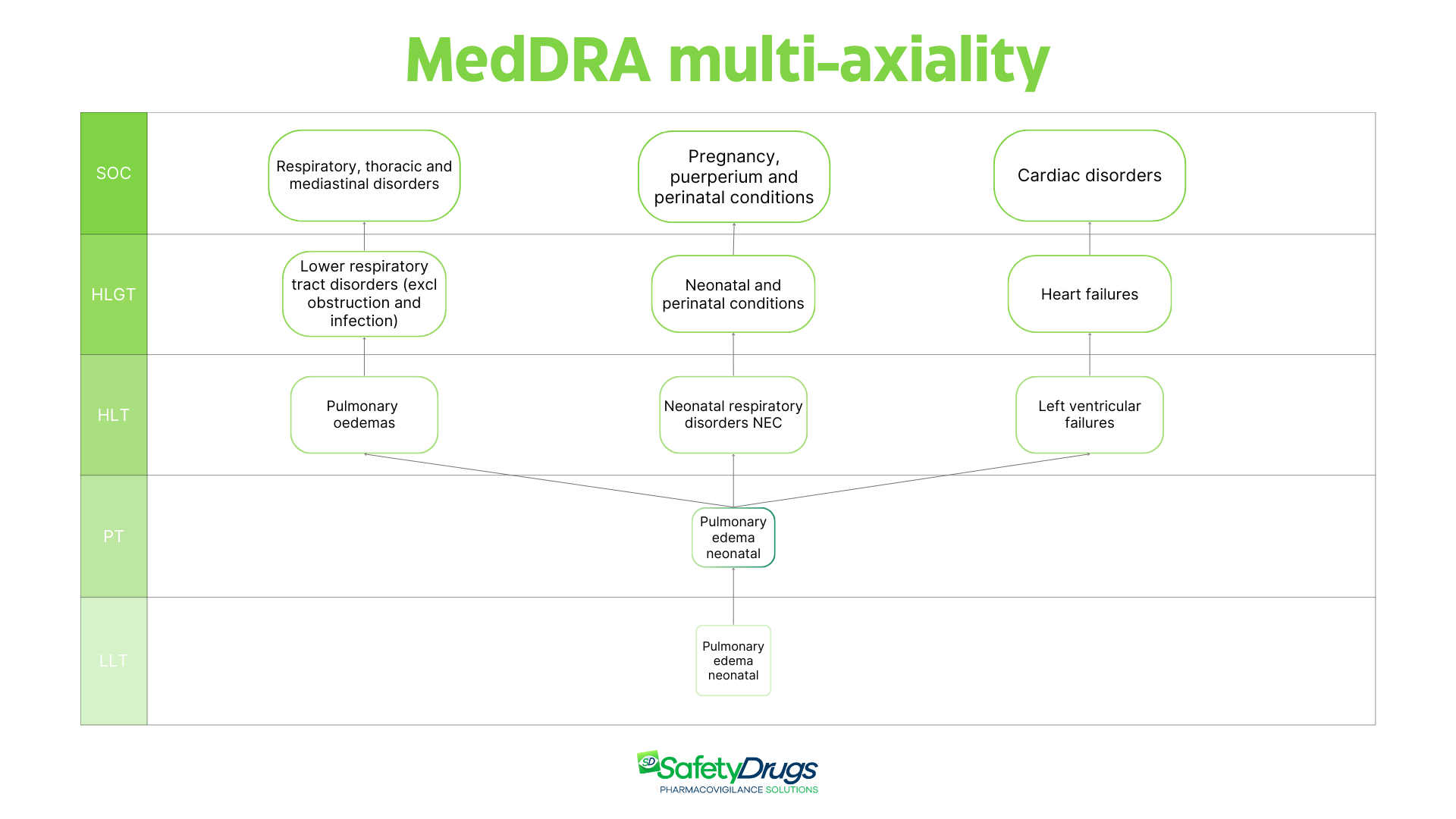
Example of multi-axiality: the term “Neonatal pulmonary edema” belongs to three different SOCs, demonstrating the complexity and flexibility of the MedDRA structure. Terms shown are based on MedDRA version 27.1.
MedDRA codes
Each MedDRA term is identified by a unique eight-digit numerical code. These codes have no intrinsic meaning; they do not convey information about the term itself but serve solely as stable identifiers to ensure accuracy, traceability, and consistency over time.
Initially, codes were assigned in alphabetical order, starting with the number 10000001. As new terms are added to the system, codes are assigned sequentially. Generally, codes are not reused, except in specific cases where minor corrections are made, such as spelling corrections or formal updates to term names. In such cases, the code may be retained to ensure continuity and consistency in pharmacovigilance databases.
SMQs (Standardised MedDRA Queries)
SMQs (Standardised MedDRA Queries) are structured sets of MedDRA terms, generally selected at the Preferred Term (PT) level, that represent specific medical conditions or areas of clinical interest. These groupings are designed to facilitate the search, identification, and retrieval of potentially relevant cases from pharmacovigilance databases.
SMQs enable more efficient and focused analyses, supporting signal detection and regulatory review activities. They are essential tools for standardizing the approach to identifying critical events, allowing for more precise monitoring and more timely risk management.
MedDRA updates: a continuous improvement cycle
The Maintenance and Support Services Organization (MSSO) releases a new version of MedDRA twice a year, in March and September. The English versions are published first, followed by localized versions, including Italian, a few weeks later.
These updates allow for:
- The inclusion of new medical conditions or emerging safety issues;
- Refinement or correction of existing terms;
- Revisions of multi-axial relationships to reflect new clinical evidence.
Updates can also occur in extraordinary cases. For instance, during the COVID-19 pandemic, the committee promptly added new terms in version 23.0.
You might be intersted in: MedDRA forced to an unexpected urgent update due to Covid-19
Why is MedDRA essential in pharmacovigilance?
The use of MedDRA is not merely a regulatory formality: it is a true quality and strategic tool in pharmacovigilance data management.
A correct and up-to-date use of MedDRA allows you to:
- Make safety reports clearer and more analyzable, reducing interpretation errors;
- Facilitate the detection of safety signals thanks to its hierarchical and multi-axial structure;
- Ensure data comparability across different markets, overcoming linguistic barriers;
- Integrate seamlessly with pharmacovigilance systems (such as EudraVigilance and FAERS).
MedDRA: the responsibility of companies engaged in pharmacovigilance
For companies involved in pharmacovigilance, keeping their systems aligned with the latest MedDRA version is an essential requirement. This involves:
- Downloading the updated files from the MedDRA portal (a valid license is required);
- Uploading the files into their pharmacovigilance systems;
- Verifying the alignment of existing historical data.
Using an outdated version can lead to regulatory reporting errors, data inconsistencies, and hinder safety analyses. Every coding error can result in a missed signal or, conversely, an unjustified safety alarm.
MedDRA and reporting quality: our support
Managing each update can be complex. For this reason, we offer a free service of uploading the new MedDRA release into the safety database on the official scheduled release date. This allows pharmacovigilance teams to focus on clinical case interpretation, confident that they are always working with an updated and compliant data set.
The conscious use of MedDRA, regularly updated and integrated into company processes, is not just a regulatory obligation but a critical factor for the quality, timeliness, and reliability of pharmacovigilance activities.







