MedDRA 28.0 has been released: the latest version of the internationally adopted medical regulatory dictionary used for coding adverse events and analyzing safety data. This biannual update introduces new terminology, hierarchical revisions, and functional improvements that reflect the evolution of clinical practice and regulatory requirements.
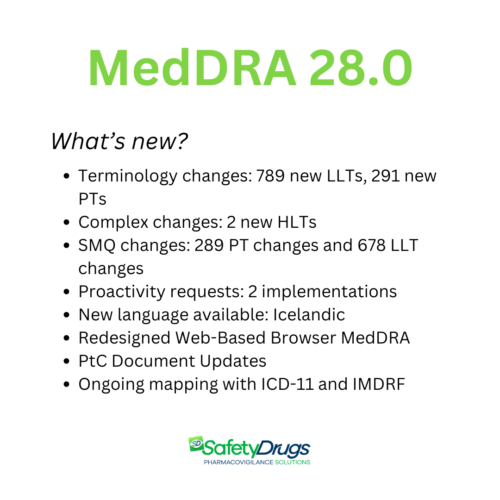
What’s New in MedDRA 28.0?
Every six months, the Maintenance and Support Services Organization (MSSO) releases an updated version of MedDRA. Changes are based on user-submitted change requests, proactive proposals, and internal reviews.
For version 28.0, 1.620 change requests were reviewed:
- 1.207 were approved and implemented
- 411 were not approved
- 2 remain pending for further evaluation in future releases
- Key updates in MedDRA 28.0 include:
- Terminology changes across all hierarchy levels
- Complex modifications
- Updates to SMQs (Standardised MedDRA Queries)
- Proactive interventions on term placement
- Expansion of available languages
- Updates to the Web-Based Browser (WBB) and MVAT tool
- Revisions to the Points to Consider (PtC) documents
- Progress on mapping initiatives
You might also like: What is MedDRA and why it is essential in pharmacovigilance
MedDRA 28.0 terminology updates
The approved changes affect all MedDRA hierarchy levels: SOC, HLGT, HLT, PT, and LLT. Key interventions include:
- 789 new LLTs
- 291 new PTs
- 23 promotions from LLT to PT
- 35 demotions
- 151 LLTs reassigned under different PTs
- 17 LLTs and 5 PTs assigned a new Primary SOC
- 1 MedDRA term variation
- 2 currency changes
- 3 complex changes
To help users identify changes between versions, MedDRA offers the Version Analysis Tool (MVAT), a web-based tool to compare any two versions, including non-sequential ones.
Complex changes
Among the most notable updates are two new HLTs:
- “Quality system issues”, under the SOC “Product issues”;
- “Rhabdoviral infections”, under “Infections and infestations”, which incorporates the previously separate HLT “Rabies viral infections”.
SMQ updates and suspensions
No new Standardised MedDRA Queries (SMQs) were added in version 28.0. However:
- 289 PT modifications;
- 678 LLT modifications were made within existing SMQs.
The development of a new SMQ related to neurodevelopmental disorders has been temporarily suspended due to methodological considerations.
Proactivity requests: enhancing classification consistency
Proactivity requests allow users to suggest refinements. In version 28.0, MSSO implemented two such proposals:
- HLT Sexuality issues: the LLT Not sexually active was moved under the PT Sexual abstinence;
- HLGT Sleep disorders and disturbances: 23 changes were applied, including the addition of the new PT Sleep-related breathing disorders.
MedDRA in more languages
With the release of version 28.0, Icelandic has been added, bringing the total number of supported languages to 24.
Translations into Bulgarian, Maltese, Norwegian, Romanian, Slovak, and Slovenian are underway, expanding accessibility and supporting the use of MedDRA in multilingual regulatory environments.
Web-Based browser: redesigned and enhanced
An important technical update accompanies this release: the MedDRA Web-Based Browser (WBB) has been completely redesigned.
New features and improvements include:
- A cleaner, more intuitive user interface
- Integrated MVAT tool
- Ability to submit translation change requests
- Display of mapping status for selected terms
- Direct submission of change proposals from the browser
- Optimized term search
- Interface and language customization
A significant step forward for those who consult the MedDRA dictionary frequently and strategically.
Updates to Points to Consider (PtC) Documents
With MedDRA 28.0, the PtC documents, published by ICH, have also been updated. These practical guidelines address not only term selection but also data retrieval and presentation strategies.
The PtC for term selection has undergone minor editorial changes to align with its companion document. Specifically, some sections related to patient outcomes were updated to ensure consistent use of “serious criteria” terminology. These changes are minimal and do not affect the practical use of the document.
More significantly, the companion PtC document has been released in version 3.0, responding to user feedback requesting more in-depth guidance on specific topics. This version updates sections 1–4 (Introduction, Data Quality, Medication Errors, Product Quality Issues) and introduces a new section 5: Manufacturing and Quality System Issues This new section provides practical guidance on coding deviations and nonconformities in manufacturing processes, including examples for real-world scenarios.
The update significantly enhances support for users operating in complex regulatory and production environments.
MedDRA and interoperability: mapping initiatives
In parallel with the document updates, several mapping initiatives are underway to improve interoperability between MedDRA and other global health terminologies.
One key initiative is the ICD-11 to MedDRA mapping project, led jointly by the World Health Organization (WHO) and MSSO on behalf of ICH. The project involves progressive mapping of ICD-11 chapters to facilitate conceptual alignment and support consistent data retrieval and coding.
Another major initiative involves the International Medical Device Regulators Forum (IMDRF). Annex E of IMDRF terminology—covering clinical signs, symptoms, and health effect codes—has been mapped to MedDRA through a collaborative effort with ICH.
The mapping is publicly available on the IMDRF website.
These efforts aim to create seamless connections across regulatory systems and terminologies, enhancing global communication and harmonization.
How to Update Your Pharmacovigilance Database to the New MedDRA 28.0 Version
Every new version of MedDRA must be incorporated into the pharmacovigilance database. Max Application offers a free service for uploading the new release into SafetyDrugs, the pharmacovigilance safety database. In this way, the pharmacovigilance team can immediately operate with updated terminology, without having to manage technical activities or incur regulatory misalignments. In the daily practice of pharmacovigilance, accurate coding and an always updated dictionary are key elements for identifying signals, drafting reliable reports and maintaining regulatory compliance.







